How drugs that hit targets for depression and kidney disease could help people suffering physical pain.
Could repurposing drugs to treat the agonizing pain of sickle cell disease help address chronic human pain in general?
Researchers have been studying sickle cell disease since 1910, when a physician in Chicago named James Herrick described the first case of it, but much of that work has focused on its causes and health outcomes and the changes it makes on body tissues and organs. Little has been done to better understand the disease’s biggest hallmark: the awful, agonizing pain.
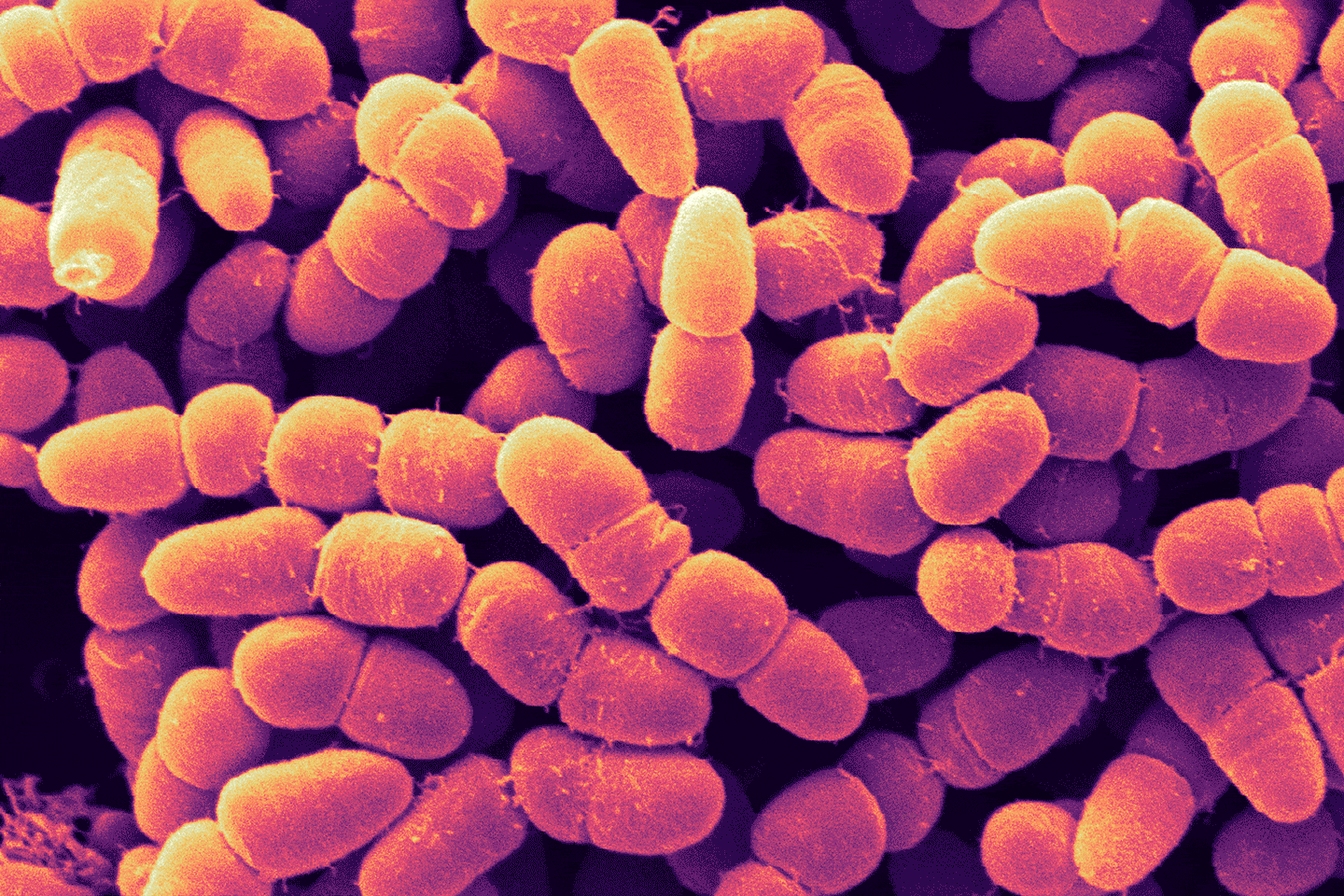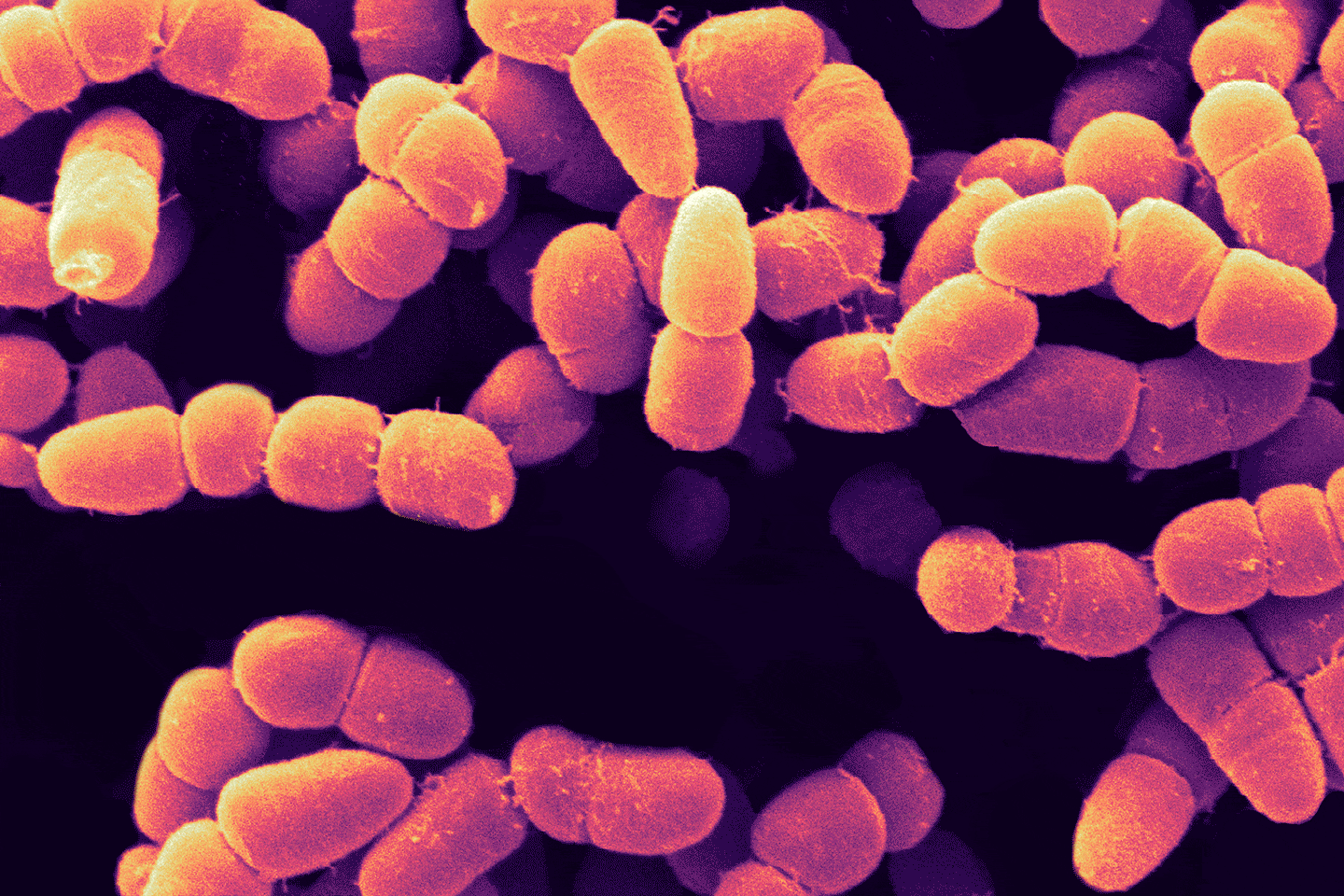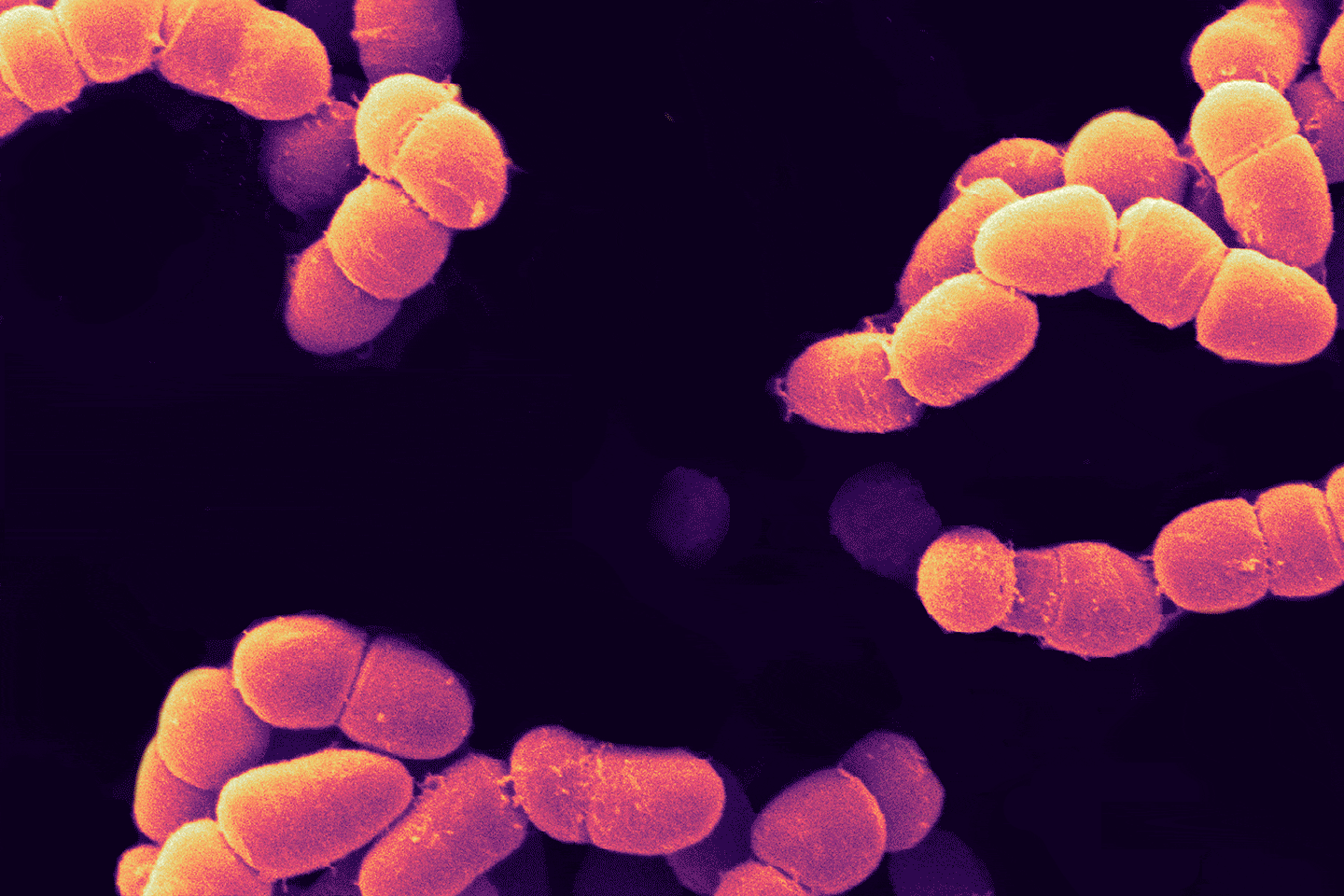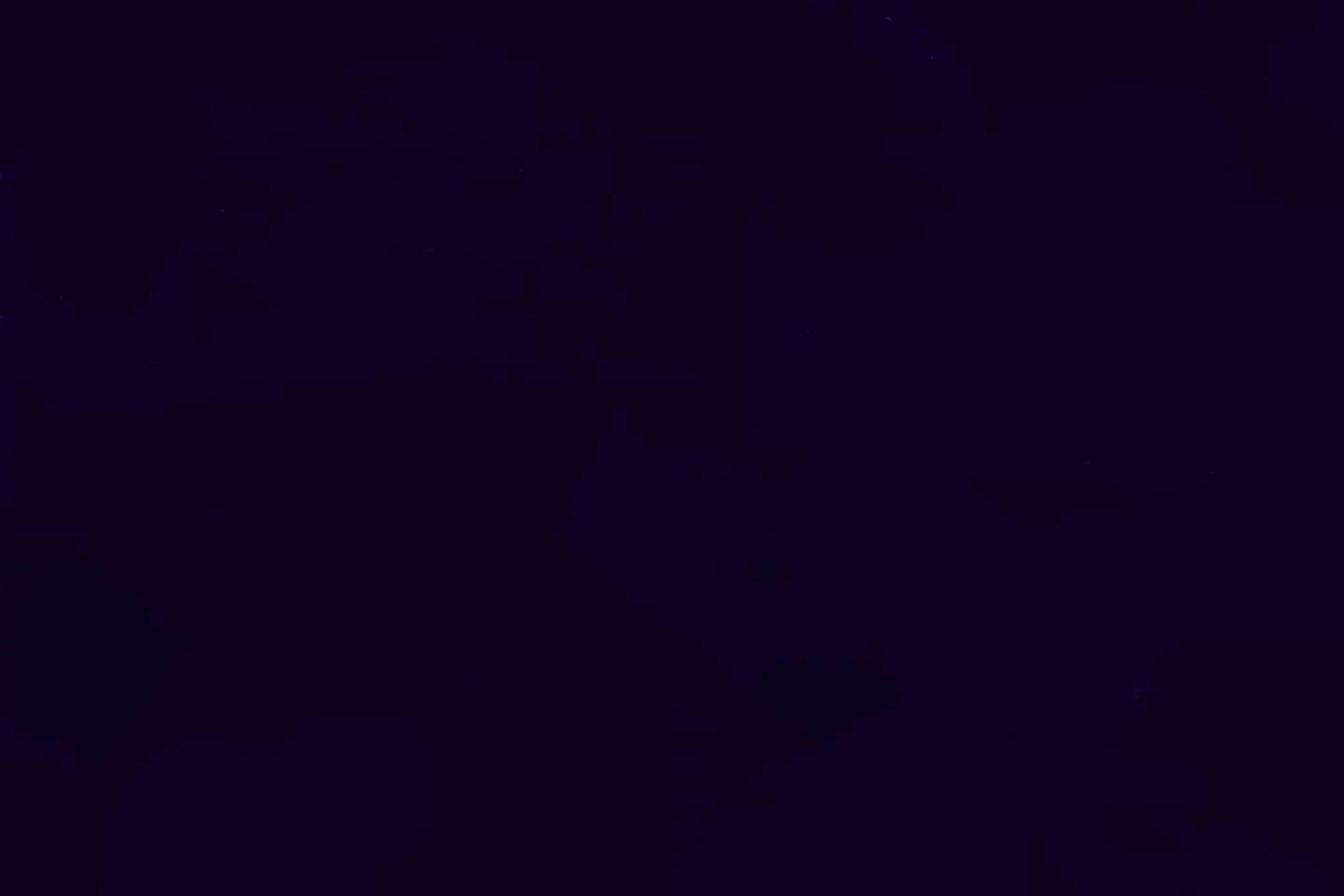
Some people have likened it to being stabbed with knives all over their body. The horrific pain occurs because their red blood cells have morphed from a canonically shaped round disk into a canoe or sickle-shaped thing. Those sickled cells have sharp, sticky corners. But so do blood vessels, and that makes the movement of the sickled cells through the bloodstream difficult. They can bunch up, like a microscopic 10-car collision, and those pile ups can block blood vessels, depriving downstream tissue of all the oxygen red blood cells are loaded with. This causes anemia and can starve the body’s cells to the point where they begin to die. But long before that happens, it can fill a person’s veins with extreme pain.
That searing pain, felt by the 100,000—mostly Black—Americans who suffer from this disease, is a prime motivator for Cheryl Stucky, a pain researcher at the Medical College of Wisconsin who has been studying sickle cell anemia since 2009. And new work from her lab may lead to a non-opioid drug for treating not just sickle cell disease but pain in general.
The discovery began in 2016, when a woman in Stucky’s lab was researching pain mechanisms and found a study showing that people who have sickle cell disease had elevated levels of a particular lipid fat molecule in their blood. That lipid, called lysoPC, activates an “ion channel” protein called TRPC5, which sits on the surface of sensory cells.
“Treatment with a TRPC5 inhibitor completely reversed the chronic pain these mice exhibited.”
The channel, in turn, sends pain signals to the spinal cord and brain. The lipid is essentially a molecule that acts like a key to open the ion channel, allowing ions like sodium and calcium to rapidly rush through and into the neuron, activating it. Stucky and her team of researchers reasoned that if this protein channel could be blocked, it would stop sending those signals and the pain neurons would not be activated.
“This other group was also studying sickle cell disease, but they were looking at how red blood cells undergo sickling,” Stucky says. “They saw elevated lipid levels that correlated with sickling events in mice and human patients. We saw their paper and wondered whether it correlated with the pain during sickling.”
Worked in mice
To test their theory, Stucky’s research team gave mice with sickle cell disease a drug, referred to as a small molecule inhibitor, that binds to and blocks the TRPC5 channel from being activated. They found that by stopping the lipid from activating TRPC5, they were able to stop the pain. The experiments worked on mice with not just sickle cell disease but migraines, chemotherapy-related pain, and surgical pain.
“I almost didn’t believe how well the TRPC5 inhibitor worked when we first tested it in sickle cell disease mice,” says Katelyn Sadler, a postdoc at the Medical College of Wisconsin. “Treatment with a TRPC5 inhibitor completely reversed the chronic pain these mice exhibited.”
Sadler says two other lab members repeated the experiment and got the same result she did, which got their minds racing as they tried to figure out first, if this analgesic effect was due to blocking TRPC5 channels in the peripheral nerves or the brain, and second, what else activates TRPC5 channels in sickle cell disease? Since lysophosphatidylcholine or LPC (the lipid) is not the only compound that drives pain in sickle cell, Stucky’s lab is trying to determine what those other factors are.
“We didn’t stop a single lipid in any of our experiments. We inhibited or stopped activity at a convergence point for lots of lipids—and likely other compounds that we haven’t identified yet,” Sadler says. “Shutting down this convergence point—TRPC5—made the pain neuron fire less.”
But that was in mice. So researchers in Stucky’s lab applied their idea to humans. They obtained some human pain sensory neurons, or balls of neurons that lie beside the human spinal cord, from organ and tissue donors and found that TRPC5 existed in those human neurons, too. Stucky also put the human version of TRPC5 into a cell line and determined that just as it did in mice, the lipid activated the protein channel in the human cells as well. In fact, she and her colleagues believe her protein inhibitor could be even more effective in relieving pain in humans than in mice, because human pain neurons have a lot more of this protein than mice neurons. While TRPC5 is expressed in about 15 percent of sensory neurons in mice, it can be found in about 75 percent of all sensory neurons in humans.

“She basically checked that this channel exists also in humans. Not everyone does that. A lot of (research) has just stayed with the mouse,” says Franziska Denk, who heads up a lab at King’s College in London that studies chronic pain.
But those wanting a non-opiate alternative for pain shouldn’t celebrate just yet. She’s got a lot more regulatory hurdles to address before her research can be turned into a sellable product.
“It’s like when you’re hoping to find treasure buried in a boat. Cheryl has found evidence that suggests the boat might be further downstream, but you still have to find the boat,” Denk says.
What’s crazy about the analgesic drug development field is that the two most common analgesics everyone uses—opioids and ibuprofen—would never pass regulatory muster today.
That said, if it were just any drug, it could take another 10 years for a product like this to get on the market, but because the U.S. government has the funding and the political will to address the opioid epidemic, it might speed up the process, Denk says. The National Institutes of Health currently fund nearly $300 million in projects involving non-opioid pain management, according to the NIH’s funding database.
“With all the money the U.S. government is putting into this, it could be faster,” she says.
What’s crazy about the analgesic drug development field, Denk says, is that the two most common analgesics everyone uses—opioids and ibuprofen—would never pass regulatory muster today. Opioids are addictive and ibuprofen causes stomach ulcers and can make headaches worse in people prone to them, she says.
“So we are trying to find a painkiller that works really well but is also super safe, and that’s quite hard to do,” Denk says.
To treat depression… and pain?
Stucky isn’t the only one focused on TRPC5. There are at least two other clinical trials currently underway involving drugs that block TRPC5, though neither aims to address pain. Cambridge, Massachusetts-based biotech Hydra Biosciences and the German pharmaceutical giant Boehringer Ingelheim are using the experimental small-molecule drug BI 135889 to inhibit TRPC4 and TRPC5 as a way of treating depression. Another, by Goldfinch Bio, uses an inhibitor with the designation GFB-887 to treat kidney disease.
Goldfinch, for instance, found that people with declining kidney function due to a rare kidney disorder called focal segmental glomerulosclerosis had elevated levels of protein in their urine that could be reduced by inhibiting the TRPC5 channel. Anthony Johnson, president and CEO of Goldfinch, acknowledged that mice bred without the TRPC5 gene seemed less fearful, an indication that the gene affects the brain.
“This target is expressed in the brain,” he says. “and so maybe there are potential benefits beyond the kidney.”
Stucky said about five months ago, she reached out to the companies working on TRPC5 inhibitors but they weren’t really interested in collaborating, which she said she understands, because even if TRPC5 inhibitors can address pain, if her data or hypothesis leads to inconsistencies in their data, it could impede the development of their drugs. proto.life spoke to Goldfinch and reached out to Hydra but received no response.
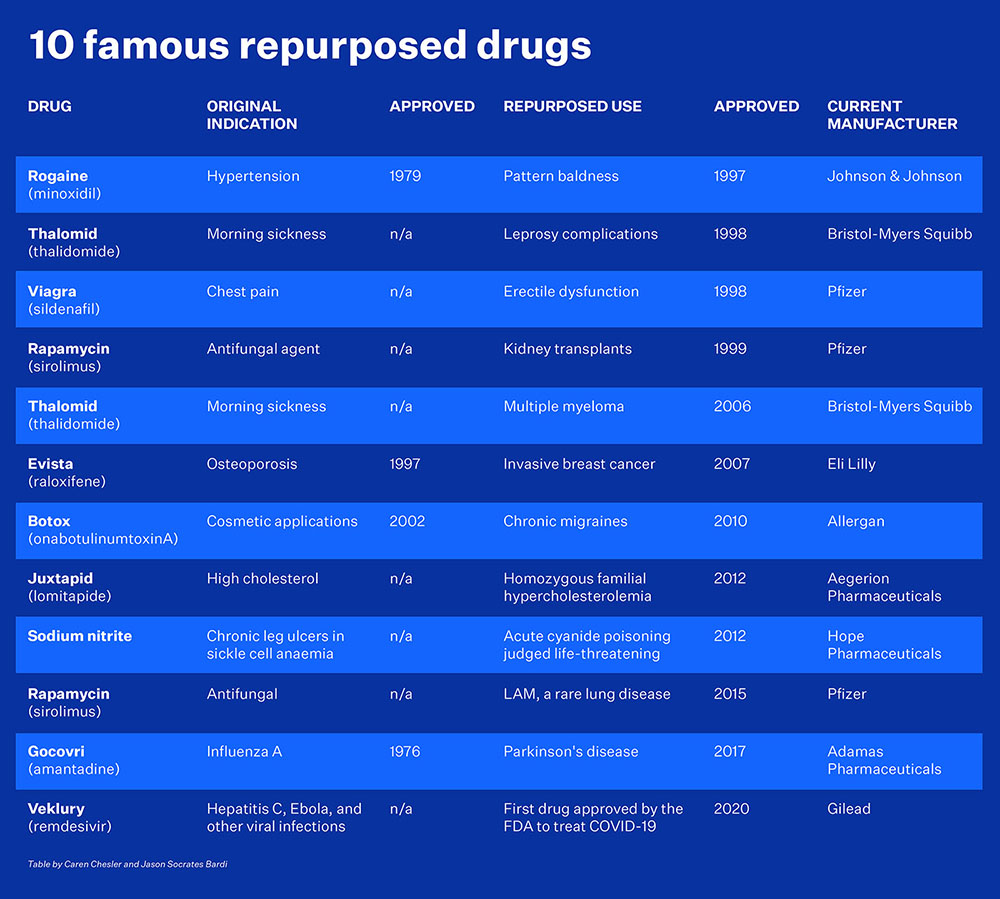
That said, she notes that botulinum toxin A or Botox was developed for cosmetic treatment of facial lines but is now used for chronic migraines, and sildenafil, the chemical name for Viagra, was originally meant to treat high blood pressure and a form of cardiovascular disease but is now used for erectile dysfunction.
“It’s actually great to do that, because you’re repurposing something that’s already gone through all these clinical trials, all the regulatory systems, and you’ve found it’s safe in humans. You can sometimes jump 20 years forward,” she says.
Goldfinch’s Johnson says biotech companies such as his typically focus on the area they believe will have the highest probability of success before looking for other opportunities.
“If the drug works in anxiety, depression, pain, that will be great,” he says. “That’s not our primary goal, but an important secondary goal is to follow all the opportunities.”
“My best hope right now is to be able to use their drug for patients with sickle cell disease after they’re through with it,” Stucky says. “Or I can go and work on making my own molecule.”
If Stucky can find a non-opioid treatment for pain, clearly sickle cell patients wouldn’t be the only beneficiaries.