Brain Disorders May Start in the Gut — So Let’s Treat Them There
Dogfish sharks produce a remarkable antibacterial compound that might halt symptoms of Parkinson’s disease.
For decades, medical students have been taught that Parkinson’s disease is primarily a movement disorder. Brain cells that play a key role in movement degenerate and die, causing tremor, stiffness, and other symptoms that can take years off people’s lives. “The old story is, ‘Parkinson’s hits the brain, god knows why,’” says Michael Zasloff, a medical researcher at Georgetown University Hospital. He remembers looking at slides of Parkinsonian brain tissue in medical school in the 1970s and seeing clearly that certain neurons had vanished.
But today Zasloff is building on recent work that suggests Parkinson’s does not, in fact, originate in the brain. Rather, in many cases it may begin in our stomach or intestines, largely as a response to infection. Clumps of toxic protein, triggered by the body’s own response to viruses or bacteria, may eventually travel along nerve cells, from the gut to the brain, over the course of many years.
If this new story is correct, a lot of other threads begin to come together. For one thing, it may explain why constipation and other forms of gastrointestinal distress are common early symptoms of Parkinson’s. It may also suggest new approaches to treatment, aimed at counteracting the disease in its early stages, before it has the chance to reach the brain. Zasloff and his colleagues are now conducting clinical trials of a synthetic compound that could prevent the build-up of toxic protein in neurons within the wall of the gut. (We have neurons in our guts as part of the “enteric nervous system,” which helps to control the gastrointestinal system.)
“It’s a new way of thinking about what might help,” says J. David Beckham, who is head of the division of neurological infections at the University of Colorado School of Medicine and is also exploring the role infections play in the nervous system. No current approach to treating Parkinson’s is based on the possibility that gastrointestinal infection might be a trigger of the disease. None aims to address the disease by way of the gut, and none is able to halt the disease’s progression.
No current approach to treating Parkinson’s is based on the possibility that gastrointestinal infection might be a trigger of the disease.
Zasloff’s work also touches on other big ideas bubbling up in medicine. Infection may play a role in triggering Alzheimer’s disease as well. Scientists who have suggested that theory believe that the body’s own immune response could be partially responsible for a build-up of toxic protein that causes damage to the brain (in that case, in areas responsible for memory). Understanding the relationship between the immune response and the nervous system thus may have far-reaching implications.
His approach to Parkinson’s also highlights the interplay between the gut and the brain, a connection that is more extensive and more important to health than researchers have generally appreciated. Scientists are now exploring the links between bacteria in the digestive system and a broad range of psychiatric and neurological disorders, including multiple sclerosis, anxiety, and depression.
But while lots of research has shown correlations between the state of our guts and the rest of our bodies, Zasloff’s work stands out because it offers a detailed mechanism for how, exactly, gastrointestinal distress can affect the brain. If problems that begin in the gut can lead progressively, along a defined set of neurons, to neurodegeneration in the brain, we could soon know much more about how interconnected these disparate systems are. And if we can decipher these systems’ crosstalk, we might finally crack the code for treating some neurological diseases.
In fact, Zasloff has an idea for a treatment, and he’s been tinkering with it since the 1980s. It all began with sharks.
Too much of a good thing
The central issue in Parkinson’s disease is the build-up of a protein, called alpha synuclein, that aggregates in neurons and causes them to function poorly or die. In autopsies of patients with the disease, researchers have found clumps of this protein in the brain but also in nerve cells surrounding the gut. Over a decade ago, the German pathologist Heiko Braak showed that as the disease progressed, these clusters were more likely to be found higher and higher up — first in the gut, then in the medulla and then in an area of the brain called the substantia nigra, which plays a critical role in movement and where the lesions long associated with the disease are located.
In 2014, Swedish researchers went a step further, providing direct evidence that clumps of alpha synuclein could actually travel from gut to brain. Staffan Holmqvist and his colleagues injected the protein into the intestinal wall of animals and tracked its advancement up the vagal nerve to the brain and its movement centers. Parkinson’s disease doesn’t necessarily progress in this manner in every patient. But Zasloff argues that frequently, alpha synuclein “gunks up the nerve cells around the gut before it gunks up the brain.”
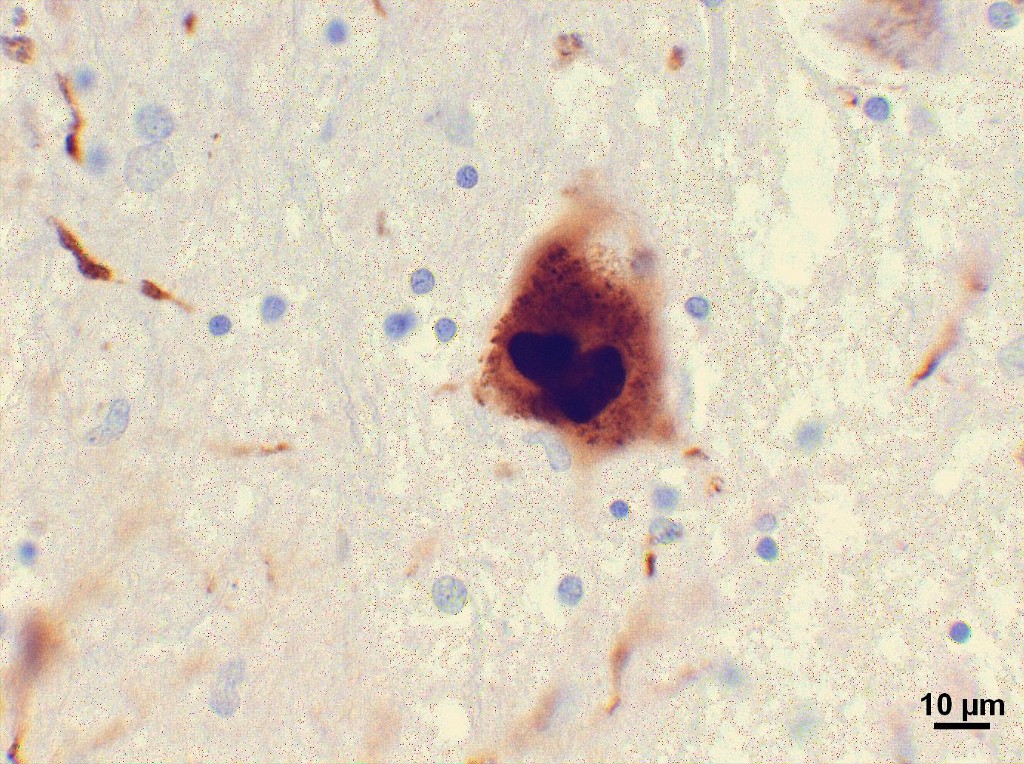
Here’s where things get tricky. Alpha synuclein also seems to have a beneficial role in the body. Researchers now suspect that under normal conditions, the protein may protect neurons from infection. Last year, Beckham showed that when mice were genetically engineered to lack alpha synuclein, they were more susceptible to viruses that invade the brain and died much earlier from them. Alpha synuclein might prevent these viruses from getting into the central nervous system and causing damage. The same may be true for neurons around the gastrointestinal tract, as well.
So let’s say you get a viral infection in your gut. Nerve cells in the area produce alpha synuclein to protect themselves and nearby tissues; Zasloff recently showed that children with norovirus produced higher levels of this protein. He also demonstrated a link between alpha synuclein and a broader defensive response. As he puts it, alpha synuclein “calls in the troops.” That means it helps to attract immune cells, including those involved in generating inflammation, to help fight the infection.
But if too much alpha synuclein builds up inside of nerve cells, exceeding their ability to clear it, it can cause damage. And that can, apparently, eventually lead to Parkinson’s disease. Zasloff argues that the key trigger could be chronic gastrointestinal infections, which cause nerve cells in the gut to produce large quantities of the protein, overwhelming the body’s mechanisms for getting rid of it. Over time, clumps of toxic protein from around the gut may travel to the brain, causing tremor and other neurological symptoms. “People don’t normally develop Parkinson’s disease till later in life,” says Zasloff. “So the system becoming overwhelmed must be a gradual thing.”
Zasloff has an idea for a treatment, and he’s been tinkering with it since the 1980s. It all began with sharks.
The idea of Parkinson’s disease propagating from gut to brain has been “one of the hottest new areas of research” for several years and has become a “new mainstream,” says Patrik Brundin, an expert on Parkinson’s at the Van Andel Research Institute in Michigan. Still, the pathway is probably not relevant to all Parkinson’s patients. For instance, in some patients, alpha synuclein may begin clumping instead in the olfactory system and propagate from there to the movement centers of the brain. In other patients, autoimmune activity may play a role in neurodegeneration.
“A lot of brain disorders are subsumed under one name, but are actually different diseases,” with different causes and different mechanisms of decay, says David Sulzer, a professor of neurobiology at Columbia University College of physicians and surgeons. He recently showed that Parkinson’s may be caused in part by an immune system attack on the brain’s own tissue. Sulzer argues that researchers must start redefining the disorder according to its many causes because “Parkinson’s is not one thing.”
Nevertheless, for patients who do experience a gut-to-brain development of the disease, Zasloff and his team have advanced the story by suggesting that viral infection could be the trigger. That is “novel,” says Brundin.
Indeed, by providing the first evidence that gut infection can lead to the build-up of alpha synuclein and therefore the potential development of Parkinson’s, Zasloff’s work may open the door to whole new type of therapy.
Seeking vindication
Most current treatments for Parkinson’s try to compensate for damage caused by alpha synuclein in the brain. Since the protein aggregates in neurons responsible for movement, which rely on the neurotransmitter dopamine, many treatments aim to replace dopamine. That relieves symptoms of the disease but does not address its root causes. However, if it were possible to clear alpha synuclein from cells, especially before it causes damage, it might be possible to slow the progression of disease. “The big hope is to find people who are at risk and put them on a medication that they could take to decrease the chances of getting Parkinson’s disease five or 10 years from now,” says Beckham.
As it happens, Zasloff has spent years studying a molecule that seems to prevent alpha synuclein from forming toxic clumps and could have therapeutic benefit. The inspiration goes way back to the 1980s, when he isolated a molecule that sharks use to defend themselves against infection. “Every animal gives you a little bit of a clue,” says Zasloff.

It was early in his career, and Zasloff was conducting genetics research that required him to remove the ovaries from African frogs and isolate their egg cells. He noticed that the frogs always seemed to heal without developing infections, even in dirty water. This was “impossible to understand based on everything I knew,” he says. So he set out to explain it, and discovered that the frogs relied on previously unknown compounds known as antimicrobial peptides to defend themselves. Soon thereafter, he realized that dogfish sharks, too, have an uncanny ability to avoid infection. Pregnant dogfish carry fetuses in their oviducts — the shark equivalent of the Fallopian tubes — for two years. Every day, they clear the waste produced by the pups by ejecting fluid into the sea and refilling the chambers with seawater that teems with bacteria. He took this to mean that dogfish must have “a remarkable system of defense” and set out to isolate whatever compounds were responsible. After years of painstaking work, in which he made “soups” of various shark tissues, like stomach, oviduct and liver, and tested them for their ability to kill bacteria, he isolated a novel compound, called squalamine, with antibiotic properties.
Zasloff had founded a company called Magainin Pharmaceuticals and attempted to commercialize an antimicrobial peptide he had isolated from the frogs. Ultimately, the effort failed: the Food and Drug Administration rejected the drug. “This was one of the more difficult periods of my life,” he recalls. In the late 1990s, however, Zasloff was determined to try again with squalamine, which seemed to have broad therapeutic potential. Indeed, his company decided to focus on testing squalamine first for the treatment of cancer, because the compound seemed to inhibit blood vessel formation, which was a holy grail of cancer treatment at the time. For a year, Zasloff says, he personally extracted half a ton of dogfish liver each week, in order to obtain enough squalamine for testing. The company got as far as Phase 2 trials of squalamine for ovarian cancer, and Zasloff says the tests were promising. Yet in 2000, the company chose not to go further. “The board decided it couldn’t compete with Genentech,” which at the time was turning heads with its own future cancer drug, Avastin. Thwarted, Zasloff eventually left the company in 2001.
Ultimately, they hope to address Parkinson’s in its earliest stages — or even identify high-risk individuals who could be treated before the disease develops.
His interest in squalamine has persisted, though. And in 2012, he began to focus on its potential as a treatment for Parkinson’s. In the lab, squalamine seemed to prevent alpha synuclein from sticking to membranes and forming clumps. In 2015, Zasloff co-founded another company, called Enterin, together with neurologist Denise Barbut and chemist Bill Kinney. They are currently enrolling patients for trials of a synthetic derivative of squalamine, which is taken orally. The goal is to address the non-motor symptoms of Parkinson’s, including constipation and sleep disorders, which can occur as alpha synuclein clusters reach the brain. Ultimately, they hope to address Parkinson’s in its earliest stages — or even identify high-risk individuals who could be treated before the disease develops. Zasloff expects the company to complete Phase 2 trials by the end of 2018, with Phase 3 potentially in 2019.
It’s too soon to say whether Zasloff’s particular compound will prove as effective for halting Parkinson’s disease as it is for protecting sharks. But as he likes to remind skeptics, quoting Michael Faraday: “Nothing is too wonderful to be true.”