What Will It Take to 3-D Print Organs?
Eventually, no one will die while waiting for a transplant. But that day is further off than you might think.
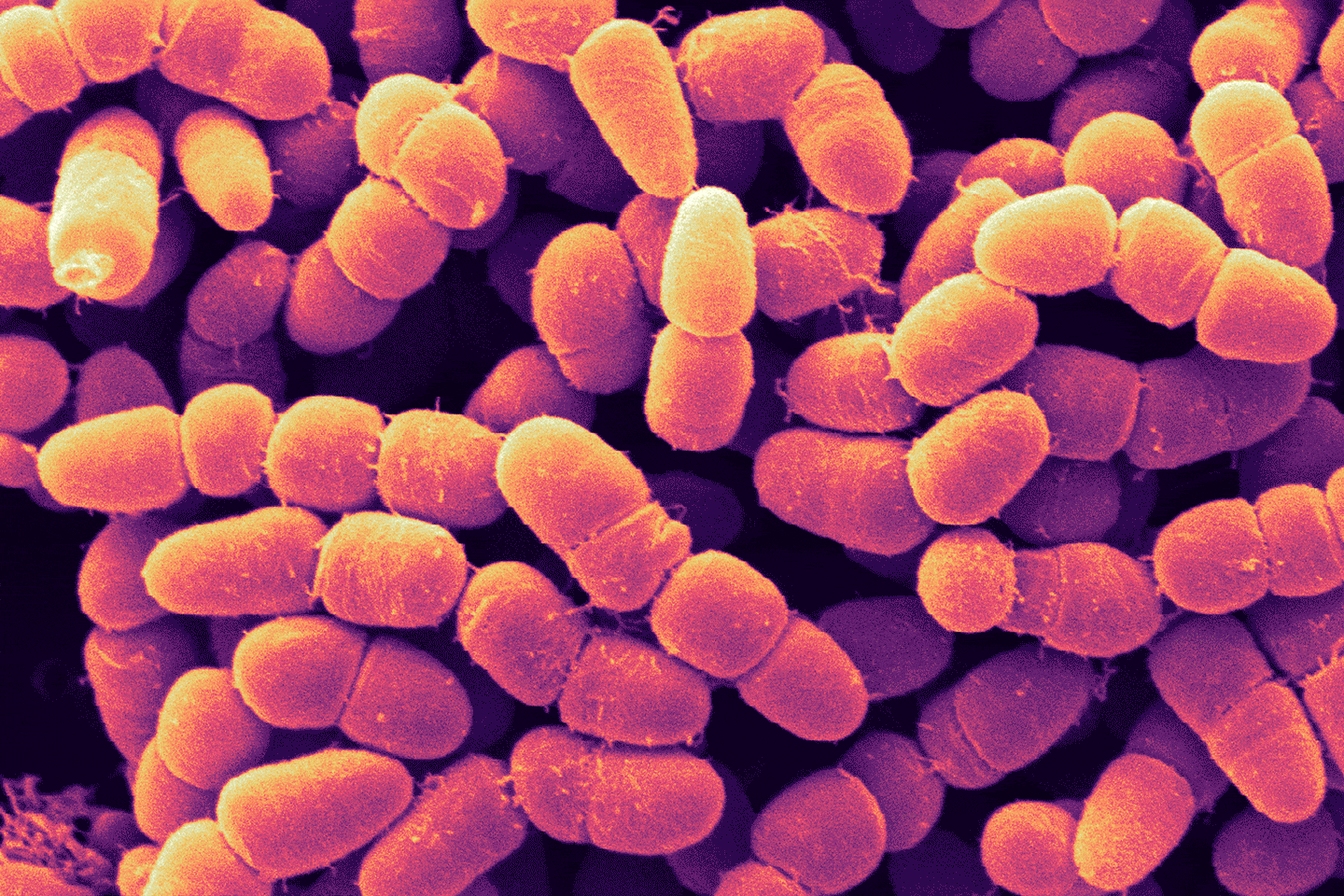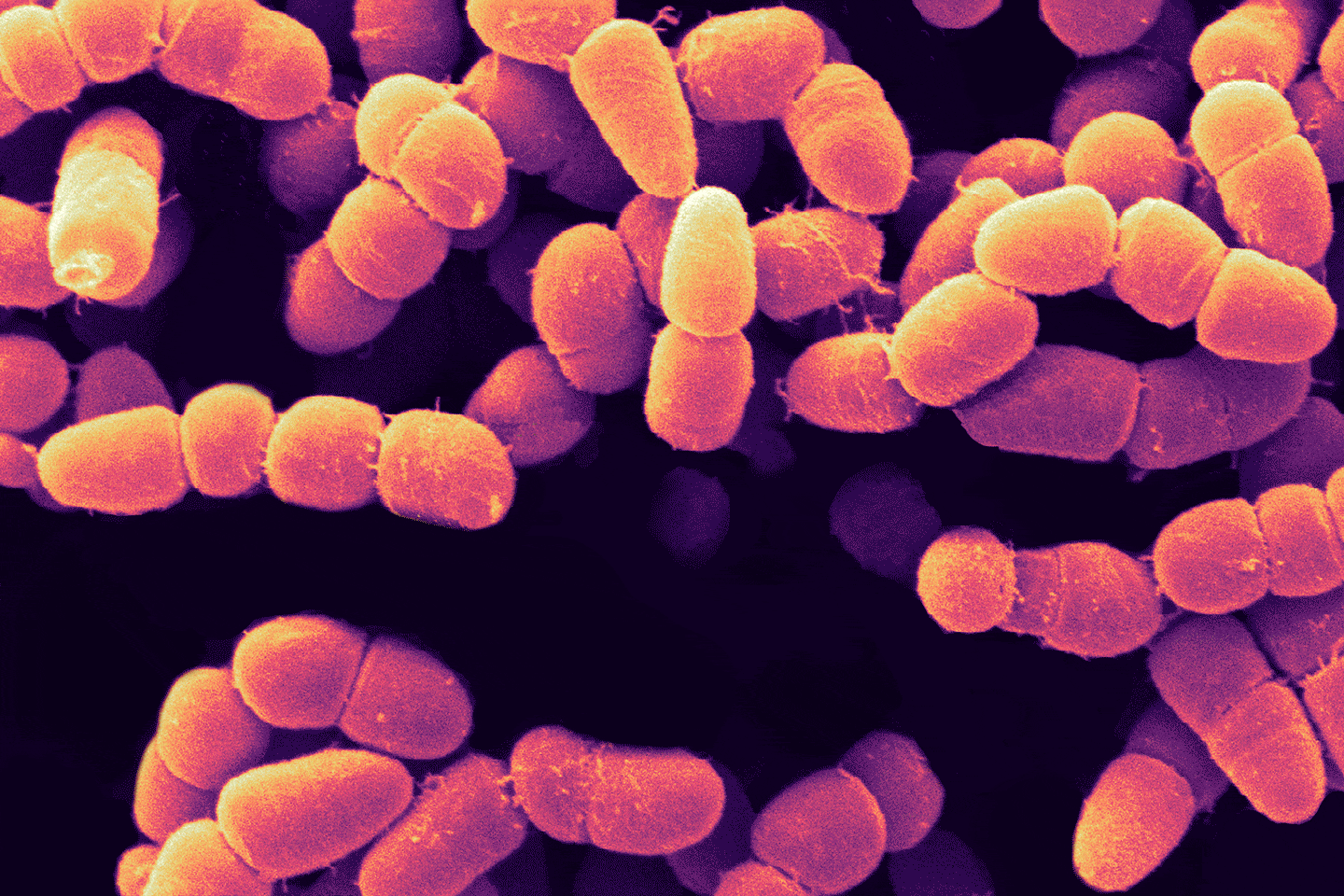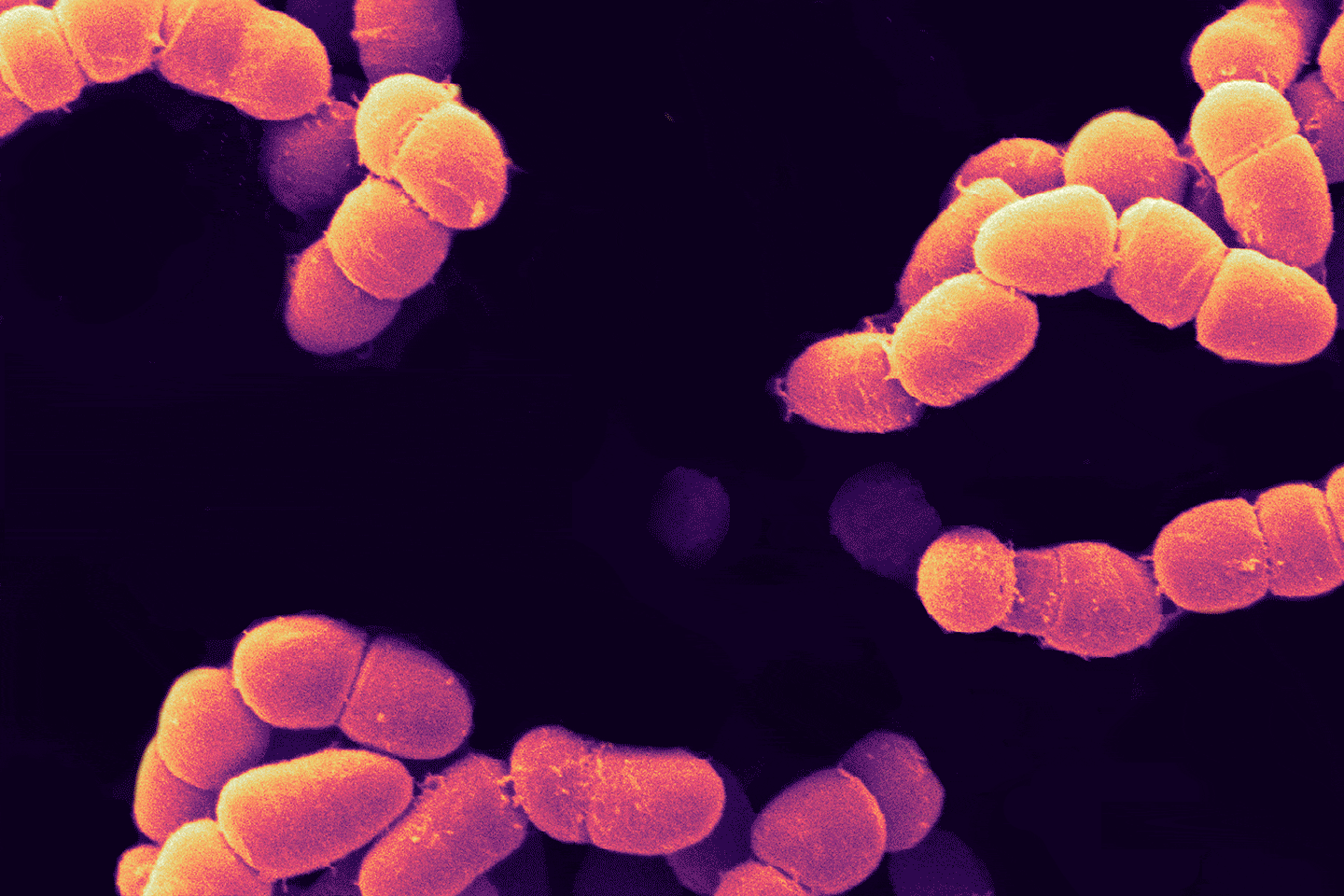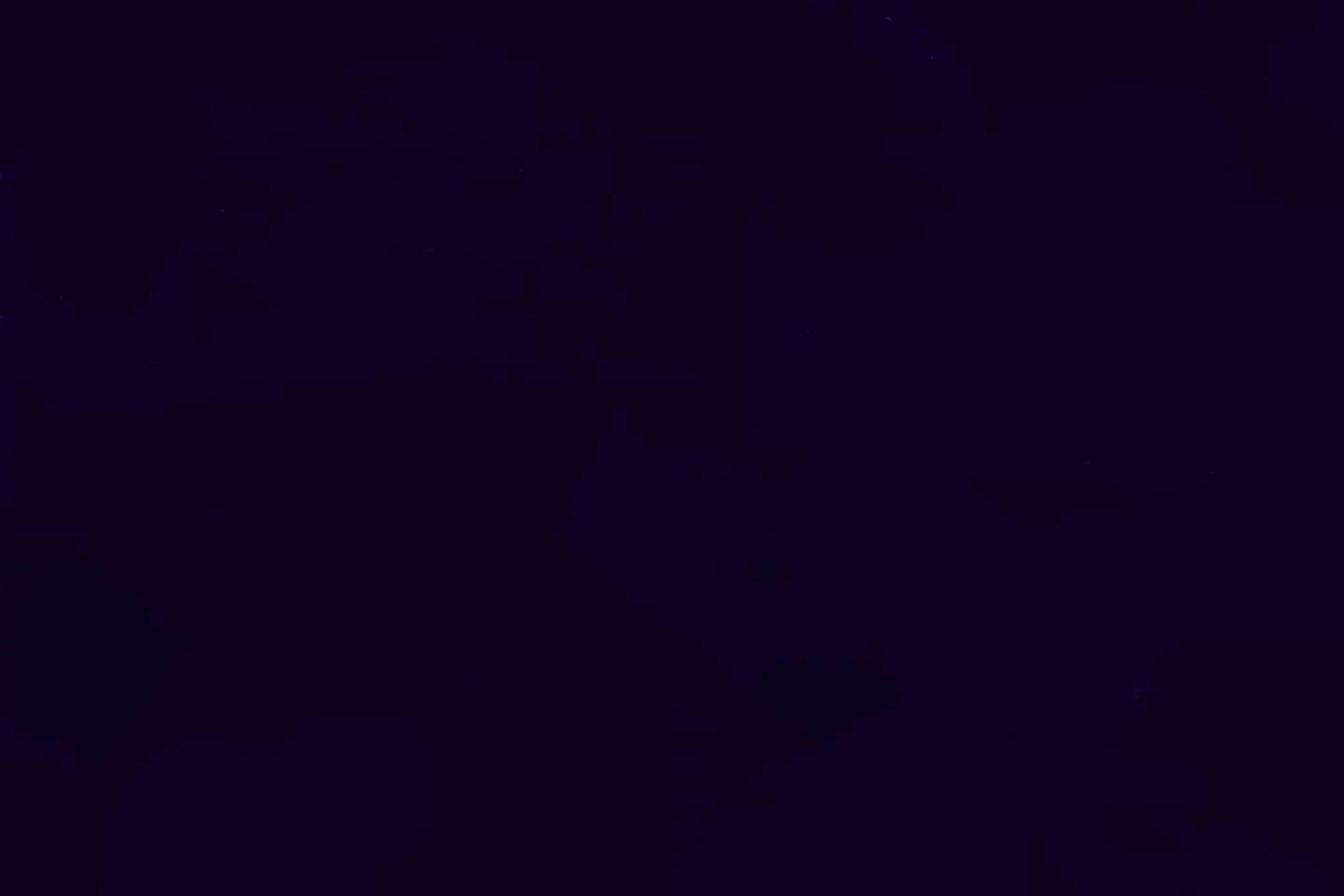
Every day in the U.S., about 22 people die waiting for an organ transplant. If scientists could 3-D print organs like kidneys, livers and hearts, all those lives could be saved. For years, people have been touting personalized organ printing as the future.
But despite decades of promising work in bioengineered bladders and other kinds of human tissue, we’re not close to having more complicated organs made from scratch. Harvard professor Jennifer Lewis, a leader in advanced 3-D printing of biological tissue, has only recently developed the ability to print part of a nephron, an individual unit of a kidney. And the segment her team can print is able to do only some of what this nephron subunit is supposed to do.
I asked Lewis what it will take to someday print a full kidney or a similarly complex organ. Current research uses either 3-D printing (also called bioprinting) like Lewis’s lab does, or what’s known as de-cell re-cell. That’s when scientists take an organ from an animal like a pig or from a human cadaver and remove all of the cells from it by applying chemicals or enzymes or freezing the organ. This leaves behind only proteins that provide a scaffolding structure on which human cells can be grown.
Lewis says that with either approach, there are three fundamental challenges to overcome, each harder than the last:
1. Physical complexity
“Kidney, liver, heart — these are just incredibly complex microstructures,” Lewis says. The kidney alone has over 20 different cell types and a million nephrons, each made of many parts and wrapped in an intricate web of tiny blood vessels. “And it’s all packed in a pretty small volume,” says Lewis. “That architectural complexity alone, that’s a challenge in and of itself for the printing process.”
Timeline to achieve:
“With bioprinting alone, it’s going to take many years,” says Lewis. However, combining the approaches of bioprinting and growing mini-organs, known as organoids, from stem cells “could rapidly accelerate that.” Essentially, you would use 3-D printing to give an organ its architecture, and grow organoids on that organizing structure. “There’s potential there within the next decade, perhaps even sooner,” she says.
2. Function
As hard as it is to build something resembling an organ, it’s even harder — and more vital — to build one that does exactly what it’s supposed to.
The kidney does two crucial things: first it filters everything out of your blood, good and bad, and then it puts the important stuff back in, leaving the toxins out so the body can get rid of them. Lewis’s lab has so far tackled the second of these functions. Last fall, her group was the first to 3-D print a working proximal tubule, one of the parts of an individual nephron that returns good stuff to the blood.
“But the proximal tubule is one subunit within a nephron that itself has multiple subunits, and then of course there’s a million of those in the kidney,” says Lewis. “While I’m super excited about that advance, it’s really a tiny step towards the entire problem.” Her lab still hasn’t tackled the other half of kidney function, the filtration.
Timeline to achieve:
Once her group can make one working nephron, then it would have to reliably produce a million of them and ensure that they function together. To do that and transplant it into a human? “I think it’s somewhere between 10 and 30-plus years, to be honest,” she says.
3. Rejection
Building an organ that the patient’s body won’t reject may be the steepest challenge of all. Most organ-printing researchers, including Lewis, use generic cells from biomedical supply companies. “It’s still not clear whether the body will treat that as from itself,” she says.
The odds could improve if you build an organ with stem cells harvested from a specific patient. But research on that technique is far off. Using repositories of stem cells from particular patients is more expensive. And it lacks one of the major benefits of using standard commercial cell lines, which is that research data is more comparable across different labs. There’s little point in using patient-specific stem cells if researchers can’t yet make a functioning organ with them. And once they can, there probably would be years of clinical trials.
Timeline to achieve:
“I still think the rejection piece is going to be the hardest to solve,” Lewis says. But she adds: “I think it is possible.”