Roswell Biotechnologies wants you to believe its new chip will revolutionize the detection of viruses, DNA, and more. But it still has to prove itself.
Imagine it’s a few years in the future, and you get the sniffles. What is it? you nervously wonder. Is this yet another variant of the coronavirus? Luckily you just bought one of those new sleek biosensor kits that attach to your smartphone. The one with a microchip that tests for more than 200 known respiratory viruses. You plug in the kit, turn on the accompanying app, and blow into a straw. Seconds later your result pops up: negative for COVID-19, but positive for a much milder rhinovirus, a common cold bug. The chip also can tell you which exact rhinovirus variant you have: the same one your daughter brought home from daycare last week.
This 200+ virus detector does not exist currently. Yet it might soon, according to San Diego-based Roswell Therapeutics, a start-up that’s developing a new biochip that hopes to detect not only dozens of viruses in a fell swoop, but also almost anything biological—enzymes, DNA, traces of drugs or vitamins in your system, pollutants in the air, and more.
Roswell’s claim is based on a re-emerging technology known as molecular electronics that was the darling of the science world 20 years ago but never lived up to its promise. This has created an aura of skepticism to some of Roswell’s claims, even as the company’s leaders insist their technology is different.
Proponents of this prototype chip, called ME 1947, believe it could be a testing laboratory shrunk so small that it fits on your fingertip. Measuring one square centimeter and looking something like a standard “Intel Inside” microchip, it has tiny rows of nano-sensor circuits embedded in a thin, gray-blue metal wafer.
But the ME 1947’s 16,000 sensors are not made of metal oxides embedded in silicon. They are built of biomolecules engineered to detect other biomolecules and to produce and read electrical signals when they interact. “Biological information naturally flows through the physical contact of molecules,” says Roswell co-founder and chief scientific officer Barry Merriman, co-inventor of the chip. “We take advantage of this by engineering our chips to pick up the interactions among them.”
“I think it’s long overdue for chip manufacturers to do something for us in biosciences.”
Let’s say Roswell wanted to create that 200+ virus chip. Their engineers would build sensor probes tipped with engineered antibodies like those the human body makes to identify and fend off, say, the SARS-CoV-2 virus that causes COVID-19, or the rhinovirus circulating in your daughter’s daycare—plus the many variants of those viruses and dozens of others. When an infected person blew into the straw and expelled virus onto the sensors, the antibody molecules would react by producing a distinct chemical-electric signal picked up by the chip’s electronic circuits. Software would then translate the signal almost instantly into a “positive” or “negative.”
“It’s a great concept,” University of Michigan biochemist Nils Walter, who is not connected to the company, recently told MIT Technology Review. Walter co-founded Alight Sciences, which is one of several companies developing a single-molecule sensor that uses light rather than electricity to read signals. “I think it’s long overdue for chip manufacturers to do something for us in biosciences.”
This week Roswell announced their first customer: Belgium-based pharmaceutical company UCB, which will use the Roswell chip technology for drug discovery to treat neurological, immunological, and rare diseases. “Roswell’s biosensor offers a view of molecular interactions not available with current tools and could increase the scale of molecular screening,” Dhaval Patel, UCB’s executive vice president and chief scientific officer said in a press release. “We’re eager to begin this collaboration.”
But will it work?
Little green men
On a zoom call Merriman showed me the tiny gray ME 1947, which sat like a speck on his index finger. Then he showed me how the chip fit snugly into a white plastic breathalyzer that he held up. The size of a mini frisbee with a plastic cylinder attached to breathe into, this was an early version of the kit that the company hopes will one day attach to a smartphone, to use at home. Right now, the device measures only a few of the 200 viruses they might include in the future.

Merriman is a mathematician turned genomics expert with a long history of working on sequencing and other molecular technologies for biological analysis—first as a former research professor at UCLA, next as a senior executive at Life Technologies (now part of Thermo-Fisher), and then at Human Longevity. He joined Roswell in 2015, a year after its founding by CEO Paul Mola, who named the company after the town in New Mexico where a UFO supposedly crashed in 1947. (This is also where the “1947” in the ME 1947 chip name comes from.) Mola’s deep pedigree in all things molecular bioscience includes senior positions at Roche, Applied Biosystems, Life Technology, and Human Longevity.
The Merriman-Mola collaboration goes back to a joint effort to sequence the first cancer genome in 2007 and later to develop technology that helped drop the cost of sequencing a complete human genome to under $1,000, among other projects.
Roswell’s basic technology is detailed in a paper recently published in the journal PNAS, which claims the tech is better than anything else available—a bold assertion considering the bio-detection and bio-diagnostic industry is worth perhaps $80 billion (numbers are a bit fuzzy), a market that’s rapidly growing and dominated by blue chip companies like Thermo-Fisher and Roche Diagnostics.
The authors back up this claim by reporting how the company has solved some key drawbacks to past attempts to fuse molecules and microprocessors. In doing so, the paper says, the chip “realizes a 50-year-old scientific vision of integrating single molecules into electronic chips to achieve the ultimate miniaturization of electronics.”
That was a reference to 1974, when two IBM engineers, Mark Ratner and Ari Aviram, first suggested in a theoretical paper that computer microchips could be built using molecules as switches and other circuit components. The technology didn’t yet exist, but for 25 years scientists tinkered.
By the late 1990s researchers were making crude molecular circuits with aspirations to do everything a silicon chip could do. Funding increased and patents were filed by the likes of IBM, Bell Labs, and Hewlett-Packard. Gushing stories in the media insisted that molecular electronics would soon be bigger than silicon, allowing chips to be denser, faster, and more powerful. And in one fabulous—and as it turns out premature—coming out party 20 years ago, Science named these embryonic efforts the “2001 Breakthrough of the Year.” Molecular electronics was meant to be the new technology for our new century—one that would make scientists and investors everywhere salivate.
At the same time, Science also issued a caveat that turned out to be more prescient than they could have guessed. “Researchers now face the truly formidable task of taking the technology from demonstrations of rudimentary circuits to highly complex integrated circuitry that can improve upon silicon’s speed, reliability, and low cost,” the magazine wrote.
Within a decade, the triumph of molecular electronics became a cautionary tale. Molecular circuit tech turned out to be more difficult to perfect and manufacture than expected. Contrary to most predictions, silicon also continued to get better, more powerful, and cheaper. By about 2010, traditional silicon electronics had won, and funding for molecular chips had shrunk. Many researchers were abandoning the field.
Merriman and Mola are both acutely aware of this troubling history. But they say one huge difference between these early molecular chips and the Roswell technology is that the molecules twenty years ago were not biological. They were made from chemicals like benzene and certain salts and acids, which engineers at the time wanted to use to replace silicon. In contrast, Roswell’s circuits use biologically active molecules that focus not on computer processing and storage, but on detection, which is something that silicon is not good for.
The stuff that dreams are made of?
The idea of using biologically active molecules as sensor-circuits is something that Merriman and Mola developed during a quest by their company to fix two persistent problems with current biosensing technologies. Mostly, these use light (photons) and chemical tags to sequence DNA and to fish out and identify viruses, hormones, and all the other tiny pieces of life—and they don’t rely on electrical signals like Roswell’s technology.
Despite great progress being made to make these traditional methods faster and cheaper, they still are not instantaneous (think about the 15 minutes you wait for your rapid covid test results). Besides that, their cost remains high, making the tech difficult to scale. Results also can be noisy and not entirely accurate.
“We tried everything to address these problems,” Mola says, “and eventually we hit on molecular electronics, which was this old vision from years earlier that a single molecule, in this case a biological molecule, could be a circuit element.”
Mola remembers a moment late one night in December 2016 when he, Merriman, and their team, after trying for more than a year, finally booted up a prototype biosensor in their lab space in Serrano Valley, a biotech cluster near San Diego. “There were wires everywhere coming out of this little chip,” recalls Mola, with each part of the process being manually run. “We had one person going in to load up the molecule, and somebody else to stop the counter while everybody else held their breath,” he says. They then checked the oscilloscope, “and we got this beautiful picture showing up,” an electronic signal. “That was a major moment, when the team saw the exact biochemical properties in the wave that we were looking for.”
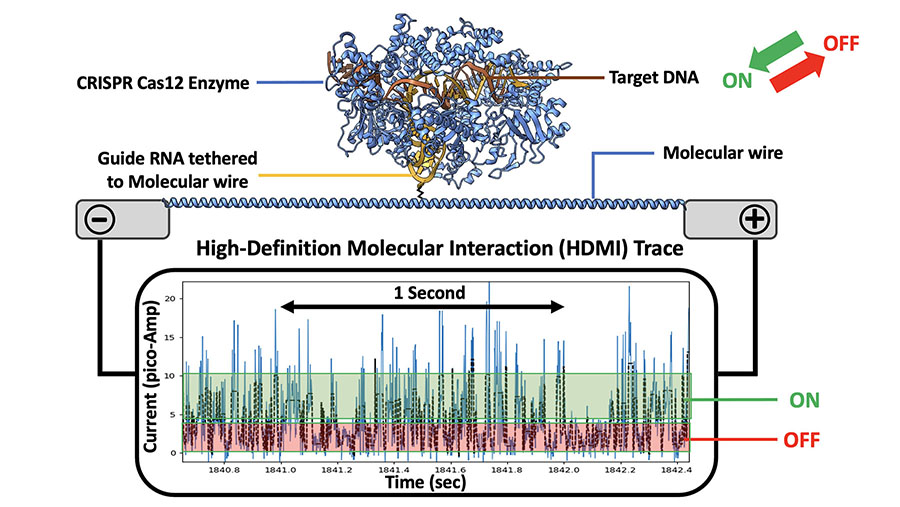
After more refinement, Roswell’s sensors now have two components—a nano-sized junction bridged with an electrode at one end to which is attached a 10-nanometer long “wire” of bioengineered amino acids with a biological molecule, like an antibody specific to a rhinovirus, attached to the far end. When the virus (or part of the virus) attaches to the antibody, the wire “reads” the signal and transmits it the short 10 nanometer distance to the electrode, which relays it as an impulse to software that interprets the results.
But Roswell’s chips can detect far more than just “positives” and “negatives.” The probes can be outfitted with the polymerase enzymes that copy the strings of A’s, G’s, C’s, and T’s in DNA, which the sensor wires can detect. “You can actually see electrically what that enzyme is doing,” says Merriman, “as it grabs a free piece of DNA and starts building the copy letter by letter. You can make a DNA sequencer this way.” The chips can also be custom programmed to test potential drug compounds to treat diseases. Another use is to identify and track complex interactions among different “omics”—genomics, metabolomics, proteomics, epigenomics, and the rest.
While all this customization sounds complicated, Merriman and Mola insist that their chips can be made using existing manufacturing processes at scale for a few dollars per unit. Geneticist George Church, a professor at Harvard Medical School and a co-author of the PNAS paper, agrees. “As far as I know, Roswell is the first single molecule transistor that is manufacturable, scalable, and versatile,” he says. Church is also a scientific advisor to the company.
A technology in search of its valuation
Roswell is hoping to start selling its 16,000-sensor ME 1947 chip by the end of the year. Powered by claims of a strong IP position—with 31 patents approved and 130 more in process, according to Merriman—the company plans to build a 100-million sensor chip. The ultimate goal is to mass produce chips with possibly billions of sensors that would be able to find hard-to-detect biomolecules. Roswell wants to raise an additional $100 million to begin to launch commercialization, says Mola, although here they have hit a snag.
For all the promise they’re claiming, the company has commitments of only $35 million beyond their initial $65 million in funding. This came primarily from strategic investors and wealthy family investment funds. Venture funds, Mola says, have been tepid.
“Maybe it’s too radical for them,” Merriman says, who acknowledged that memories of the meteoric “2001 Breakthrough of the Year” hype and the subsequent fizzle of molecular electronic dreams of the past aren’t helping. Mola wonders if part of the investor drag has to do with diversity. He is African American and was part of a recent article in Stat that pointed out the paucity of Black CEOs in biotech and among investors.
“This technology game is hard and very competitive, and investors in this arena are very savvy.”
Also challenging are lingering memories of the infamous California company Theranos, another company that promised big things for biological testing with tech that could go small, although Roswell’s technology is completely different from anything Theranos claimed.
“This technology game is hard and very competitive, and investors in this arena are very savvy,” Eric Schadt noted in an email, “and they have been burned severely—Theranos—so maybe they’re more cautious.” Schadt is the founder and CEO of Sema4, a precision medicine company, and the founding director of the Icahn Institute for Genomics and Multiscale Biology at Mt. Sinai in New York City.
Competition also is becoming fierce in the molecular sensing space, with numerous companies developing light-based sensors and a few, like San Diego-based Cardea Bio, developing sensors that use electrical signals, but with different engineering systems than Roswell.
Later this month the company plans to announce a second customer that Mola cannot yet name, one that develops therapeutics using bioreactors. These are vats that house enzymes or cells used to turn biochemicals into drugs and other products. Merriman adds that Roswell will work to develop a custom chip to monitor the subtle biological processes that occur in bioreactors and need to be carefully watched. He says they will be paid in milestone payments if they hit certain targets.
The cool investor reception is one reason that the company chose to publish their tech earlier this year. This got them some attention, but so far it hasn’t attracted the venture money they had hoped for. Nor has the fact that the company cut 30 percent of its employees in February helped— though Mola says that is related to the company’s growth to prepare for a shift from R&D to marketing and business development.
If they can raise enough money or find more customers, Roswell plans to first concentrate on selling the ME 1947 to researchers and to the pharmaceutical industry. Next, they want to move into the new and rising field of epigenetics by designing chips that can detect the methylation of DNA—a non-genetic modification of our bodies’ genes that can activate or turn them off. “Our sensor is sensitive to whether DNA is methylated or not,” Merriman says. “If there’s a methyl group there, it makes a different current pulse.”
Eventually Roswell hopes to be a supplier to diagnostic and sequencing companies. “We want to work with the Roches and the Abbotts of the world to be the supplier of their chips,” Mola says. “So, we’ll say: ‘Okay, here’s the chip made to your configuration. Now, you deploy whatever chemistry you want.’”
Ultimately the company dreams of launching a consumer version of their device like the 200-virus breathalyzer attachment for future smartphones that could tell the difference between things like coronavirus and a common cold bug. They also envision a world long sought by the quantified-self movement and advocates of precision medicine, where healthy people are constantly measured and monitored for a range of “omics” and other factors that are unique to them before they get sick. This data could be used to create a baseline profile of a person’s overall health—everything from vitamin levels to biomarkers for inflammation—that could be monitored to guide a person’s diet or to look for early indicators of disease.
In a way, the Roswell moniker is apt as the company struggles to make its case against skeptics because few names are more synonymous with steadfast belief in the face of overwhelming evidence to the contrary. There are many who continue to believe in the existence of a UFO crashing in New Mexico in 1947 even though the far more likely explanation was U.S. military Cold War espionage equipment testing—a story long since owned up to by the U.S. government. But no number of studies will ever convince people who believe in aliens that the Roswell, New Mexico UFO crash site isn’t real. The burning question for Roswell the company is: Will their PNAS study and support from leading researchers convince investors that their chip is for real?
It’s now up to investors to believe—even in a moment when the biotech funding juggernaut of recent years is faltering—that the truth is out there.
Editor’s note: This story was updated on 6/14/22 to reflect the fact that Roswell’s initial funding was $65 million, not $60 million as originally reported.